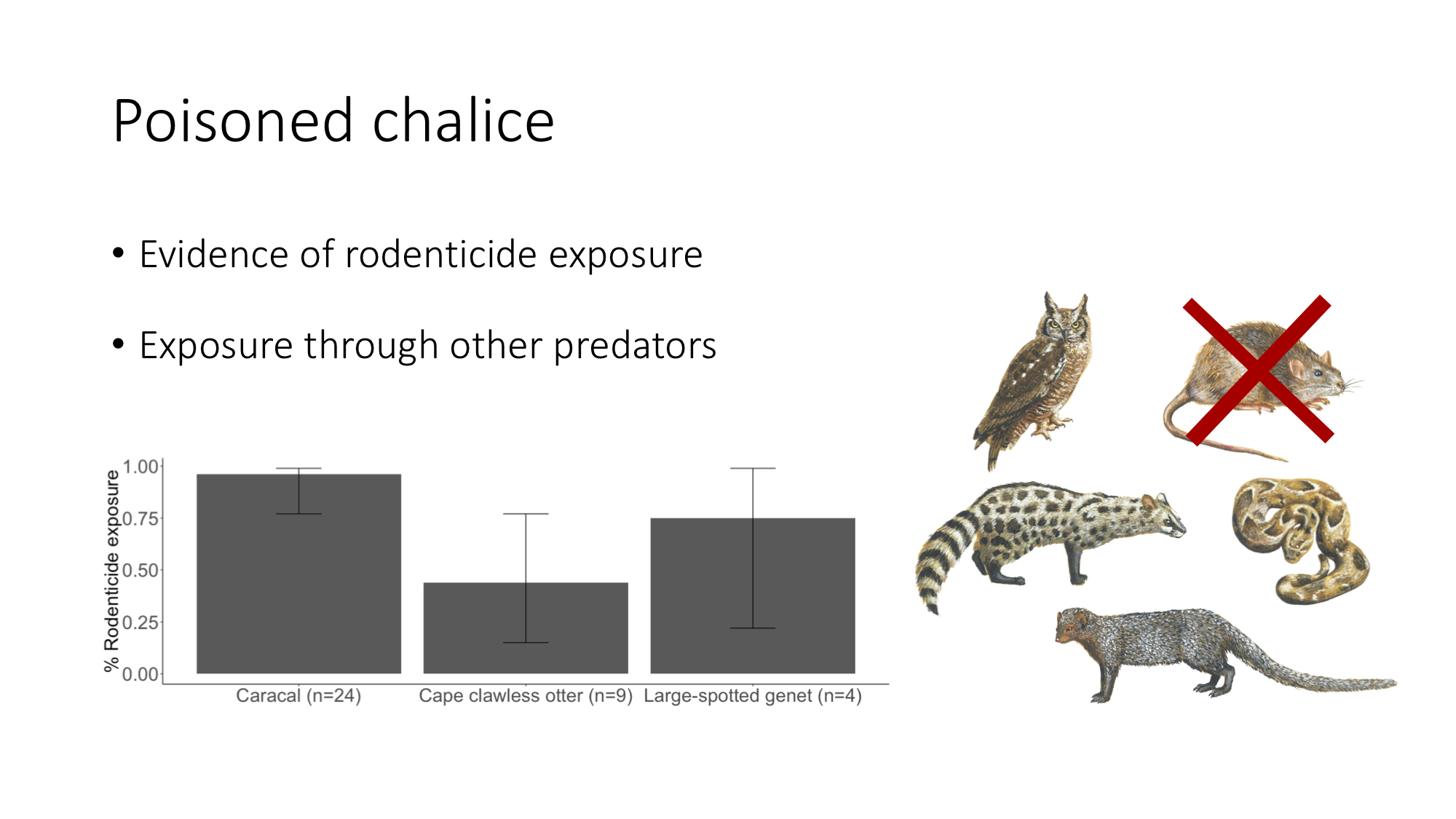
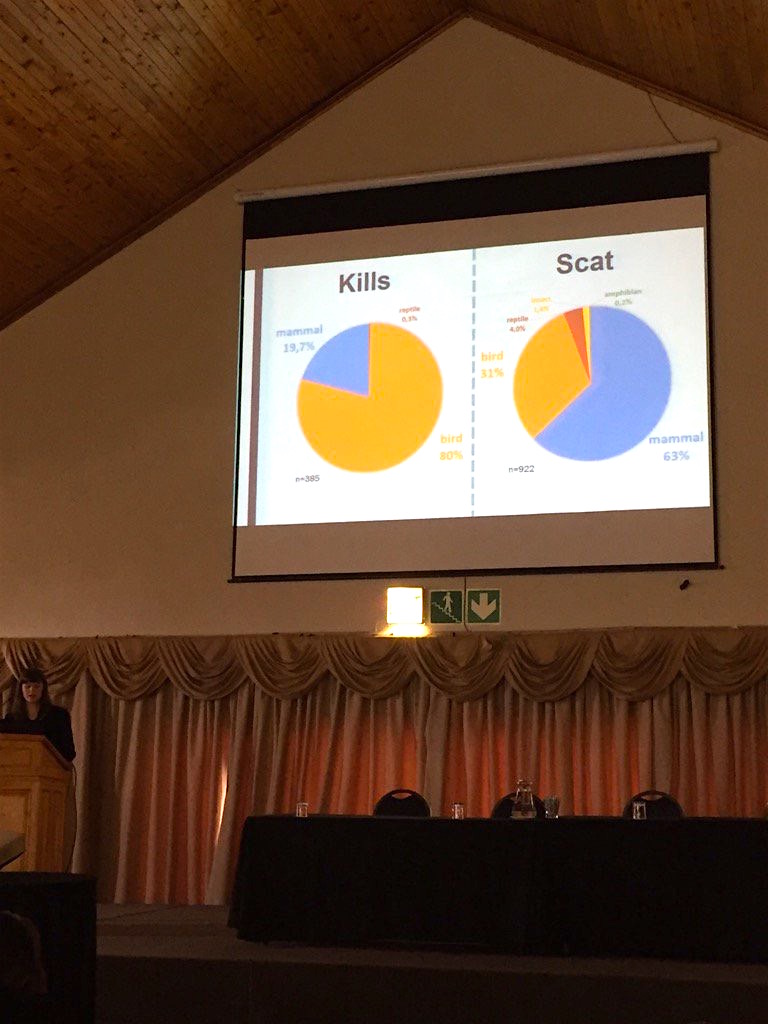
Blog post archive
Welcome to the No Free Lunches blog! These posts follow the work of Gabriella Leighton, a PhD student at UCT, working with the Urban Caracal Project to understand how urbanisation affects the foraging ecology of Cape Town caracals. For the original personal blog visit: urbancaracaldiet.wordpress.com. Here is an archive of previous posts:
-
July 2019
- Jul 10, 2019 Blog post archive Jul 10, 2019
- Jul 3, 2019 Trip report: Spanish adventures in ecotoxicology Jul 3, 2019
-
May 2019
- May 8, 2019 Urban Caracal Project goes to Spain! May 8, 2019
-
December 2018
- Dec 20, 2018 Starting that stable isotope stuff (finally) Dec 20, 2018
-
September 2018
- Sep 17, 2018 URBIO Conference 2018 Sep 17, 2018
-
May 2018
- May 21, 2018 The big ‘upgrade’ May 21, 2018
-
September 2017
- Sep 16, 2017 SAWMA symposium 2017 Sep 16, 2017
- Sep 7, 2017 Impressions: hair identification Sep 7, 2017
-
June 2017
- Jun 13, 2017 Of mice and museums Jun 13, 2017
-
May 2017
- May 29, 2017 When predator becomes urban caracal prey May 29, 2017
-
March 2017
- Mar 15, 2017 “Oh look, another cape cormorant”: some trips to the museum Mar 15, 2017
-
January 2017
- Jan 31, 2017 Two charismatic caracals' favourite snacks Jan 31, 2017
-
November 2016
- Nov 3, 2016 A few more caracal lunches, and some mystery claws! Nov 3, 2016
-
October 2016
- Oct 3, 2016 Appreciation post Oct 3, 2016
-
September 2016
- Sep 26, 2016 SAWMA symposium 2016 Sep 26, 2016
-
August 2016
- Aug 31, 2016 Some (very) preliminary diet findings Aug 31, 2016
-
June 2016
- Jun 1, 2016 Caracal diet field trips Jun 1, 2016
-
May 2016
- May 31, 2016 No such thing as a free caracal lunch May 31, 2016